SoftMolecular®
SoftMolecular is an intuitive workflow solution for molecular genetics laboratories that allows geneticists and pathologists to create customized protocols and derive data from automated instruments.
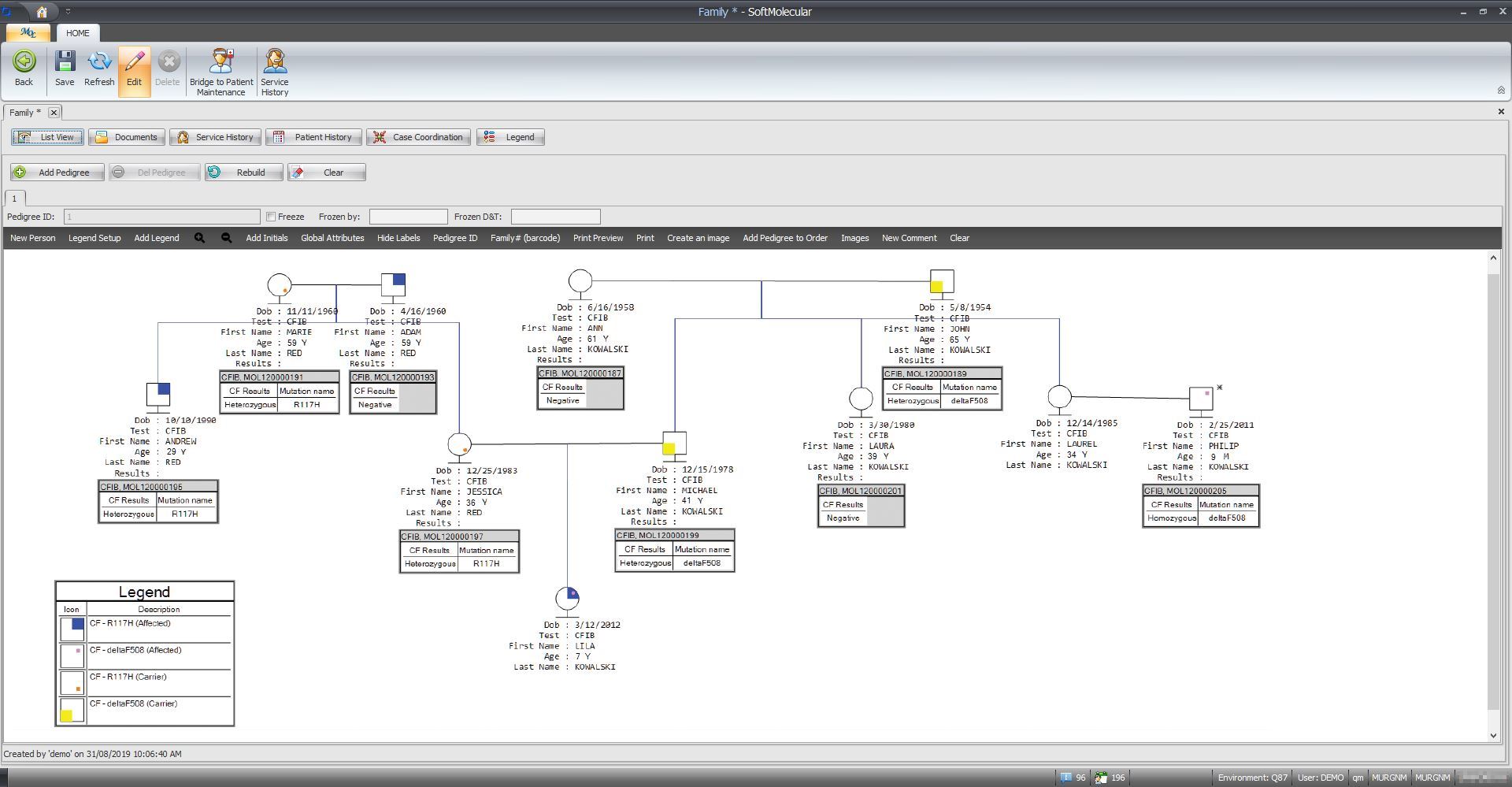
SCC Soft Computer’s SoftMolecular helps pathologists, geneticists, and forensic scientists automate workflows, manage data, and analyze molecular tests, reducing the effort needed to interpret findings and generate reports. This advanced genetics platform supports clinical and research applications of Next Generation Sequencing (NGS) including targeted panels, Whole Exome Sequencing (WES), and Whole Genome Sequencing (WGS), among other molecular technologies. SoftMolecular is a robust, user-friendly solution that allows users to easily design and streamline their own workflows, interface with automated instrumentation, and manage cases. SoftMolecular is part of Soft Computer Consultants’ (SCC’s) Genetics Information Systems Suite, which supports communication with other genetics LIS solutions.
Variant Identification
SoftMolecular enables easy identification of genetic variants, allowing users to provide their clients with clear reports.
Automation
SoftMolecular automates many functions, reducing the errors caused by manual data entry.
Rules-based Alerts
SoftMolecular generates automatic alerts based on rules defined by the user.
Features and Benefits
SoftMolecular integrates discrete, structured genomic data into the client’s electronic health records (EHRs).
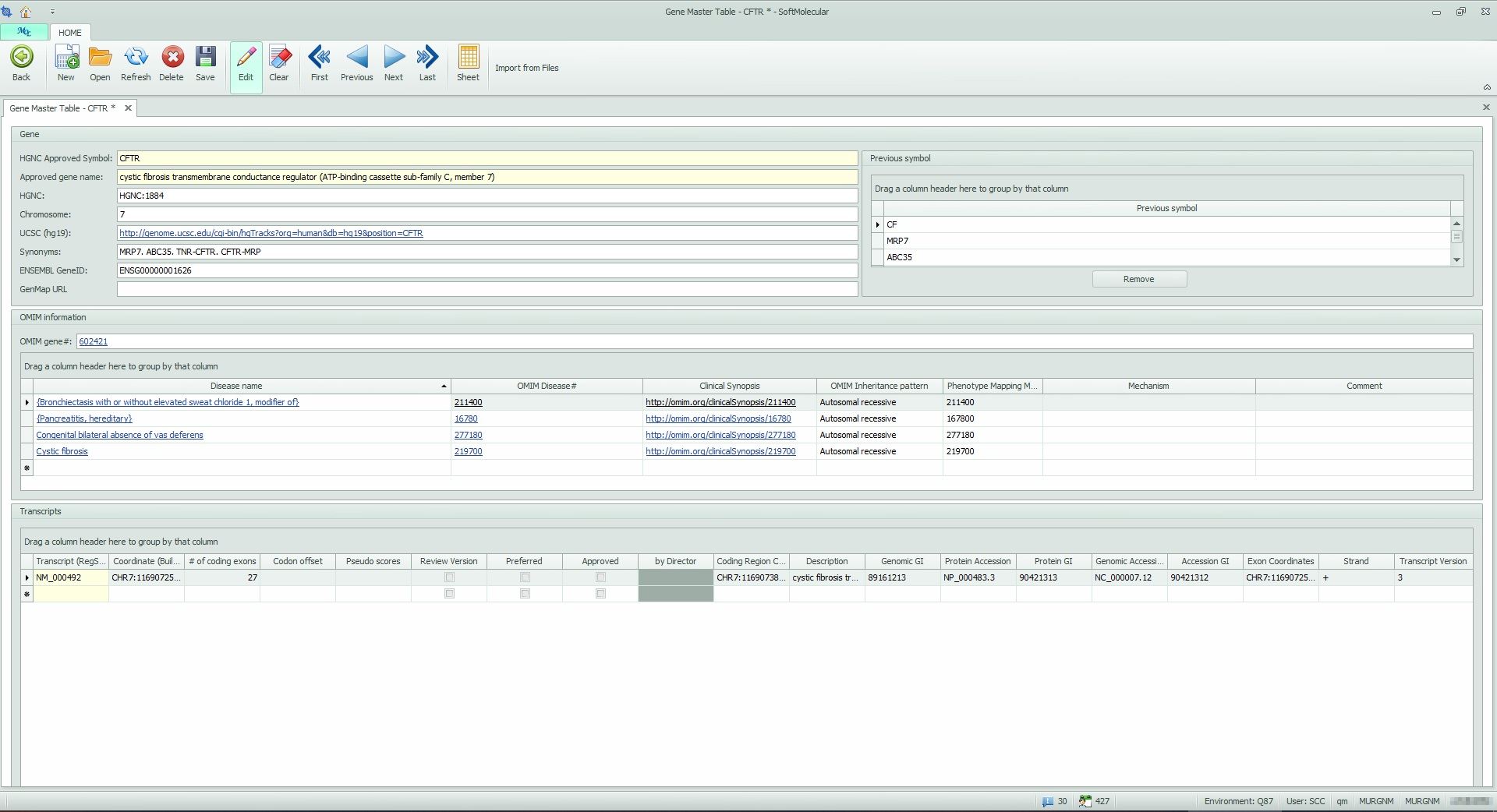
SoftMolecular supports the generation of variant databases by categorizing variants, creating versions, and linking to relevant databases like NCBI and OMIM. This feature facilitates the entry, management, and interpretation of variants. It also allows users to capture variant information from internal databases as well as hyperlinks that connect to external databases via the internet. This capability allows users to view reference material that contributed to interpretations at the single-gene level. SoftMolecular also provides versioning of genetic information, which allows users to track the source material used for an interpretation when new information affects the interpretation or variant category.
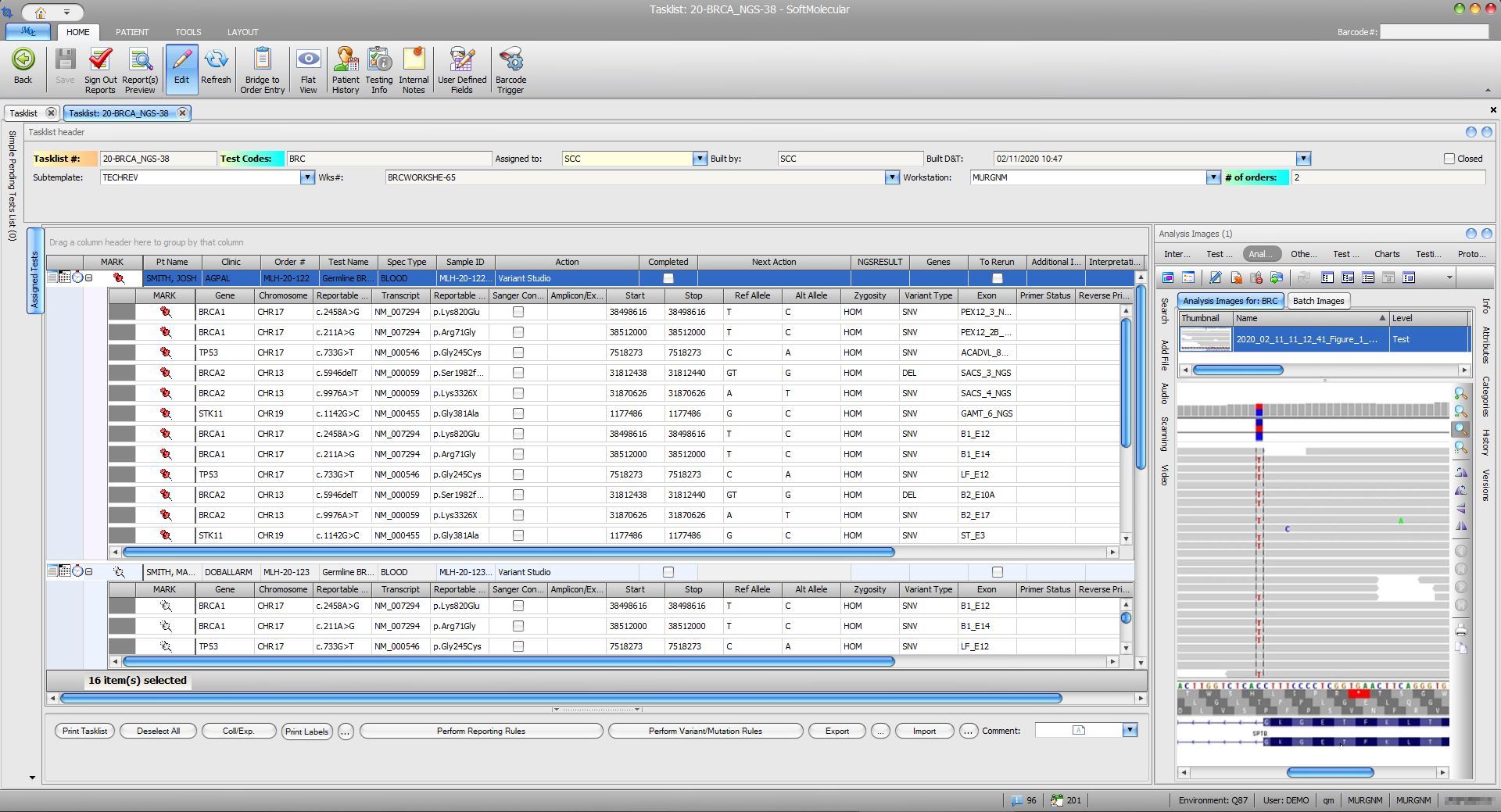
SoftMolecular provides easy identification of reportable variants via next-gene sequencing (NGS), including panels, WES, and WGS. The NGS Portal allows users to view and store DNA sequence data in addition to electronically sending this data to external databases for analysis. It can also receive results via the NGS Console. Furthermore, SoftMolecular can also be configured to interact with existing bioinformatics pipelines and external annotation services via file exchanges. This feature enables concise reporting that only covers the desired results and provides access to pertinent internet references.
SoftMolecular can be configured to integrate with the NGS-Console, which enables the user to supervise NGS pipeline execution to analyze sequencing data performed by third-party bioinformatic tools. NGS-Console also provides a seamless link between SoftMolecular and analytical pipeline execution.
SoftMolecular seamlessly interfaces with instruments to allow for easy sequencing and fragment analysis. This feature replaces manual data result entry, reducing the resource investment and error rate. As a result, it increases the accuracy and efficiency of data entry. SoftMolecular also includes the web-based WebMOL user interface, which supports specimen and testing workflows. It interfaces with sequencing and fragment analysis instruments like DNA extraction and quantification systems, single and multi-plex PCR analysis, liquid handers and robotic systems, and sequencing systems.
SoftMolecular includes images and other graphics on patient reports, providing them with a more professional appearance for clients and patients.
Users can re-run entire batches or single samples by assigning them to the next pending batch with a single mouse click. This feature allows users to easily manage repeat tests, avoiding the delays and mismanagement that often occur with manual processes. The ability to minimize turnaround time also improves client satisfaction.
SoftMolecular enables accurate documentation controls by allowing users to access documents by batch and patient. In addition, these documents remain linked for historical or investigative purposes. Users no longer need to sort through manual files or logs to identify trends in control data, thus improving quality control (QC).
SoftMolecular calculates master mixes and deducts volume from inventory automatically. This feature reduces the need for manual calculations and inventory control, resulting in error-free calculations and more effective inventory management.
SoftMolecular provides alerts and notifications based on user-defined rules, which can use parameters from the client, patient, test, and result. This feature reduces the need for communication via paper and telephone, ensuring the timely delivery of critical information like instructions. It also reduces the risk of errors and inefficiencies that often occur when personnel are interrupted at inopportune times.
SoftMolecular provides automatic testing for reflexes and reruns across different technologies. This feature eliminates errors from manual test ordering, reducing test turnaround times.